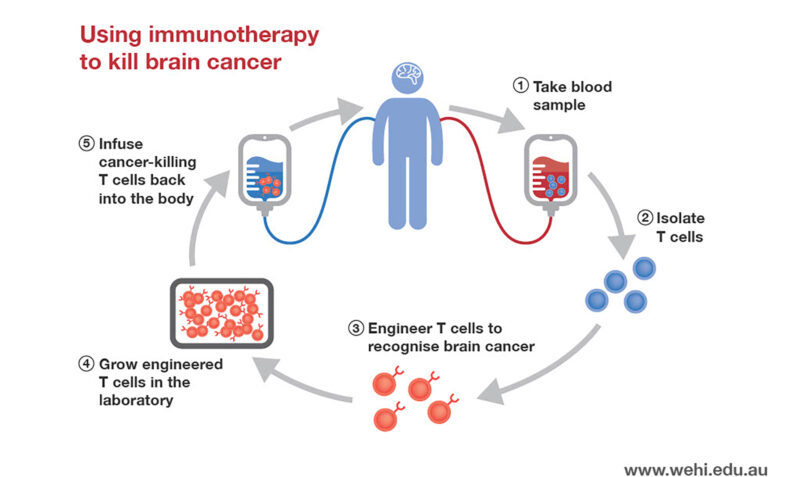
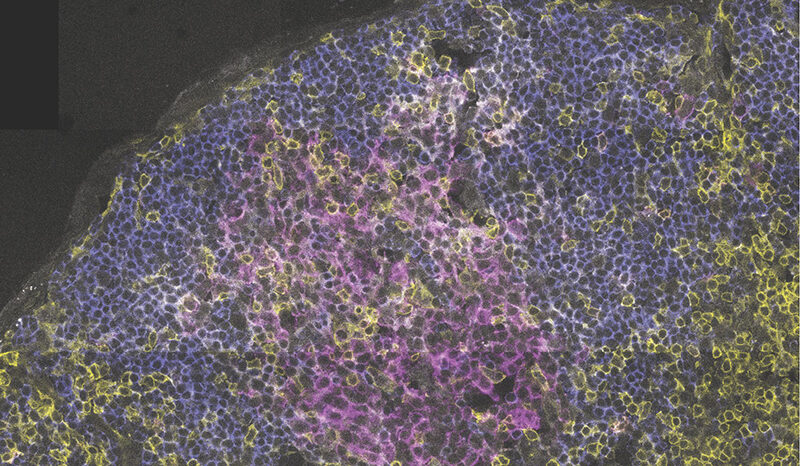
An aggressive type of brain tumour
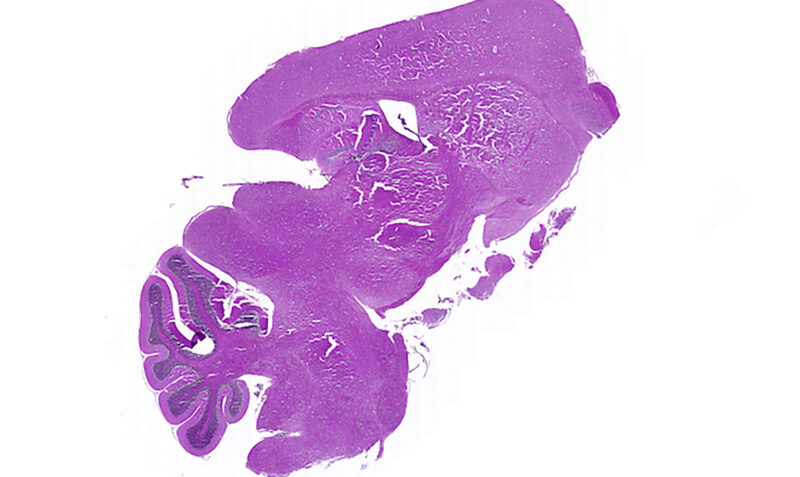
Usually occurring in children aged five to seven years old, Diffuse Intrinsic Pontine Glioma (DIPG) is an aggressive type of brain tumour that affects 20 children in Australia each year.
The fast-growing tumour forms in the part of a child’s brain responsible for vital functions like breathing, swallowing and movement, meaning it is unable to be surgically removed.
Children diagnosed with DIPG are unlikely to survive a year beyond diagnosis and there is currently no treatment for this devastating condition.